As Ph Increases What Happens to the Hydrogen Ion Concentration
When pH pass 7 and get closer to 14 it is basic. In the pH scale as H ion concentration increases pH values decrease.

8 3 The Ph Scale Sl Youtube Understanding Chemistry Scale
The hydrogen ion concentration decreases by a factor of 10 so the pH increases by 1 from 16 to 26.

. High concentrations of hydrogen ions yield a low pH acidic substances whereas low levels of hydrogen ions result in a high pH basic substances. It is an inverse relationship. What is a high hydrogen ion concentration.
PH scale based on the Hydrogen ion concentration. Where H IS CHCI d What happens to pH as the hydrogen ion concentration decreases. As the concentration of H increases.
As pH increases above 70 the hydrogen ion concentration decreases and hydroxide ion concentration increases. Hydrogen ion concentration increases 3 times. It is neither acidic nor basic and has a pH of 70.
If the hydrogen concentration increases the pH decreases causing the solution to become more acidic. Conversely the fewer hydrogen ions the higher the pH. As the Ph increases the hydrogen ion concentration decreases.
What solution has the highest concentration of hydrogen ions. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale Figure 1. Each pH unit represents a 10-fold change in concentration.
Which solution has the highest H. PH is a measure of hydrogen ion concentration H which actually exist as H3O Hydronium. For larger hydrogen ion concentrations then the pH of the solution is 7.
The hydrogen ion concentration in a solution with a pH of 3 is two times greater than the hydrogen ion concentration in a solution with a pH of 5. Thus Figure 30-3 shows that the alveolar ventilation rate. Thus as the hydrogen ion concentration increases hydroxide ion concentration falls and vice versa.
What Happens When You Increase The Concentration Of Hydrogen. When pH is at 70 the concentration of hydrogen ions and hydroxide ions is the same. What will happen to the pH of a substance if the concentration of hydrogen H ions increases from 10 108 to 10 106.
Therefore the more hydrogen ions present the lower the pH. PH -log 10 a H Where a is the activity. The concentration of lactic acid increases.
This happens when an acid is introduced. For H 108 pH 8 For H 106 pH 6 Answer link. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale Figure 1.
A decrease in pH below 7 shows an increase in acidity hydrogen ions while an increase in pH above 7 shows an increase in alkalinity hydroxyl ions. Why do you think the blood pH stayed neutral while the same amount of lactic acid in water was very. The hydrogen ions there are the more acidic the solution is which means that pH get closer to zero.
Which solution has more H ions. Not only does the alveolar ventilation rate influence H concentration by changing the Pco2 of the body fluids but the H concentration affects the rate of alveolar ventilation. In general the pH of an acid solution will be lower as the solution is more concentrated.
Apr 17 2017 It will decrease by 2. Does pH change with concentration. Therefore if we move from 7 down the pH scale toward 0 the concentration of hydrogen ions in a solution increases 10-fold with each 1-point jump.
As the hydronium ion concentration increases what about the pH. The pH of solution is defined as - logH. What happens to H as pH decreases.
Hydrogen ion concentration increases 1000 times. Since the concentration of the hydrogen ions is often very low ion activity is considered as equal to the concentration of hydrogen ions. Conversely the fewer hydrogen ions the higher the pH.
As the concentration of hydrogen ions in a solution increase the more acidic the solution becomes. Increased Hydrogen Ion Concentration Stimulates Alveolar Ventilation. As the concentration of H increases.
What happens to blood pH as the concentration of lactic acid increases. Then the pH is the logarithm of the inverse of the hydrogen ion concentration. Hydrogen Ion Concentration pH A pH of 7 is neutral.
What happens when pH of solution increases. Each pH unit represents a 10-fold change in concentration. Question A solution of hydrochloric acid with a concentration of 2 gdm 3 has a pH of 13.
An acidic solution grows when the concentration of hydrogen ions in the solution increases. Chemistry Acids and Bases pH 1 Answer Monzur R. The blood pH decreases.
A decrease in pH below 7 shows an increase in acidity hydrogen ions while an increase in pH above 7 shows an increase in alkalinity hydroxyl ions. Hydroxide ion levels increase gradually and the solution becomes more basic like acid or alkaline. How does the pH of a solution change as the hydrogen ion concentration increases.
If the pH of a solution increases from pH 50 to pH 80 what happens to the concentration of hydrogen ions in the solution. Last Updated on Fri 07 Jan 2022 Medical Physiology. Hydrogen ion concentration decreases 1000 times.
Calculating Hydrogen Ion Concentration Watch on. O pH decreases pH increases O e Determine the hydrogen ion concentration of a solution with pH-32. Rosariomividaa3 and 18 more users found this answer helpful.
This means that a low pH value represents a high H ion concentration acidic solution and a high pH value represents a low H concentration basic solution. This means if there is a change in pH in one unit the pH concentration will change by 10 fold. The logarithmic scale of pH means that as pH.
The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale Figure 1. As the hydronium ion concentration increases what about the pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale Figure 1.
It is neither acidic nor basic and has a pH of 70. If the hydrogen concentration decreases the pH increases resulting in a solution that is less acidic and more basic. The pH of any fluid is inversely related to the hydrogen ion concentration H.
Increased H results in decreased pH which is termed acidemia and decreased H results in increased pH termed alkalemia. A decrease in pH means more hydrogen ion and an increase of pH means less hydrogen ions. Hydrogen ion concentration decreases 3 times.
H 610 M Round to six decimal places as needed Determine the hydrogen ion concentration of a solution with pH 74. Therefore the more hydrogen ions present the lower the pH. PH is a measure of hydrogen ion concentration H which actually exist as H3O Hydronium.

The Answer To Our Last Mcat Challenge Was A Average Hematocrit Is 47 For Males And 41 For Females Can You Answer Today S Mc Mcat Biology Teacher Biology

Pin By Susan Castello On Physical Science Ms Hs Chemistry Lessons Teaching Science Chemistry
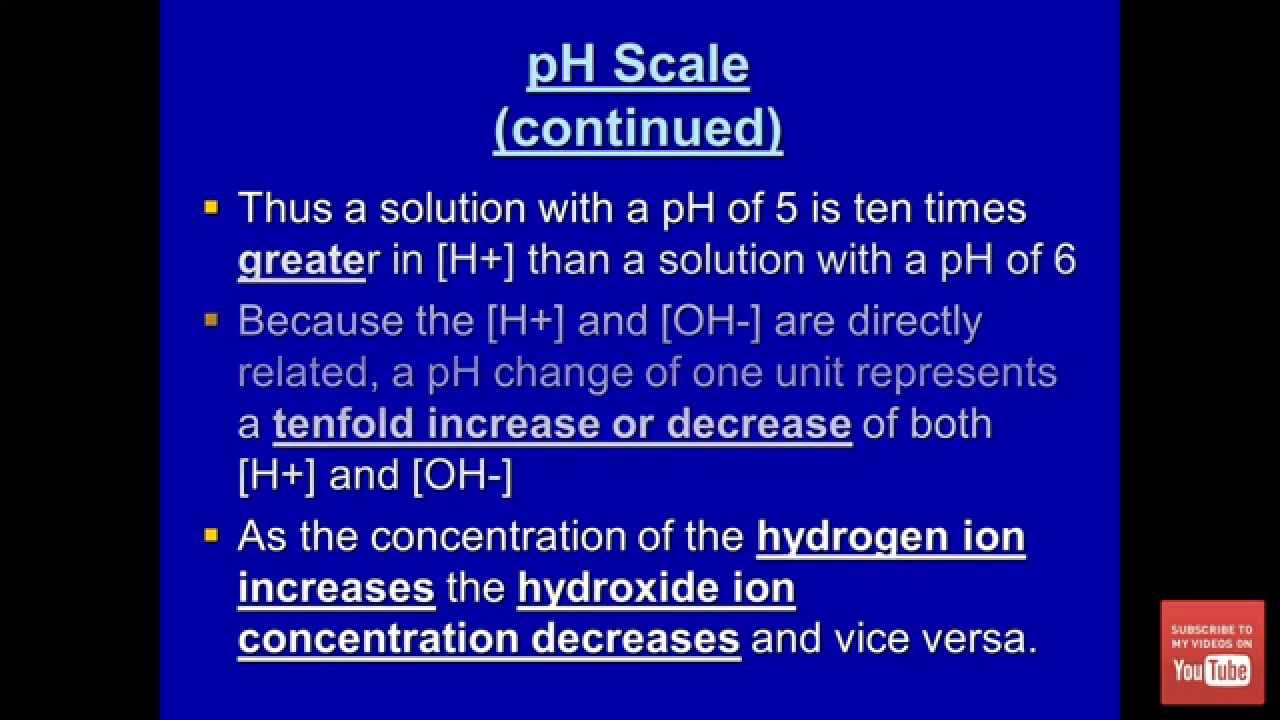
Acids And Bases Explained Teacher Created Presentation Presentation Teacher Teaching

Water Parameters Pet Advice Fish Tank Animal Companions

Even If You Re Not A Chemist You Ll Doubtless Remember Learning About Acids Back In School They Re Teaching Chemistry Chemistry Classroom Physical Chemistry

Acidosis Acidosis Dissociation Anesthesia

Ph And Poh Crash Course Chemistry 30 Chemistry Worksheets Crash Course Homeschool Science

Mcat Biology Topic Mcat Study Mcat Biology

Pin By Sea Bird Scientific On Ocean Acidification Ocean Acidification Environmental Science Lessons Environmental Education

Acids Bases And The Ph Scale Quiz Teaching Chemistry Quiz Base

Bio Is Life Biochemistry Barron S Ap Biology Workbook 5th Biochemistry Notes Biology Notes Science Notebook Middle School

7a C02 And Ocean Ph What S The Connection Matter Science Invention And Innovation Teaching Chemistry







Comments
Post a Comment